Most often, an infection that ascends from the lower genital tract causes pelvic inflammatory disease (PID). Either an acute or chronic form is possible. Pelvic peritonitis, oophoritis, tubo-ovarian abscess, endometritis, salpingitis, and parametritis are among the conditions it might induce.
The actual frequency is unknown, however in the UK, 1.7% of women between the ages of 16 and 46 receive a diagnosis each year, while 15% of Swedish women receive one during their lifetime. It can have major long-term consequences, making it clinically significant.
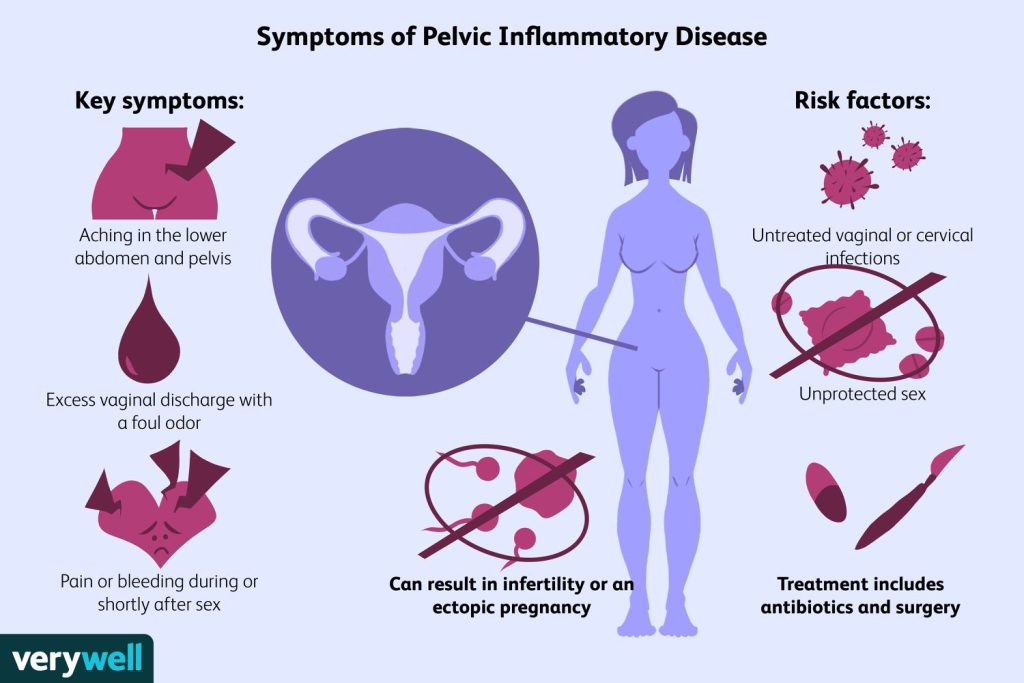
- Aetiology: One-fourth of cases in the United Kingdom are caused by the STIs Neisseria gonorrhoeae and Chlamydia trachomatis together. Other commonly encountered anaerobes in the vagina, such as Prevotella, Atopobium, and Leprotrichia, as well as Gardnerella vaginalis, have all been implicated, however they are not STIs. Additionally, connected to PID is Mycoplasma genitalium.
- One uncommon reason is genital tract TB.
- Occasionally, intra-abdominal infections—such as appendicitis—can directly spread and induce PID symptoms. Reports have also indicated hemogenous spread.
Clinical features
Symptoms of pelvic inflammatory disease (PID) - Most often bilateral lower abdomen pain, though subacute pain is possible
severe dyspareunia. - Menorrhagia, PCB, and IMB are examples of abnormal vaginal bleeding.
- Unusual discharge from the vagina.
- A backache.
- fever and systemic sickness.
- Occasionally, nausea or vomiting.
- Intense thirst.
- maybe without symptoms
Signs - Higher than 38°C fever.
- Usually bilateral, lower abdominal soreness.
- Bimanual examination: discomfort in the adnexa and/or cervical region.
- Pelvic fullness.
- infected cervical discharge
Complications of pelvic inflammatory disease (PID)
Subfertility and ectopic pregnancy risk increases significantly with even a small delay in beginning treatment. Subfertility, ectopic pregnancy, and persistent pelvic pain are all less common when antibiotics are used early. - Of women with PID, 10–20% have Fitz-Hugh–Curtis syndrome.
- Tubal damage is doubled by recurrent PID, which is an infertility cause. Risk for one episode is 8%; for two episodes, it is 19.5%; and for three or more, it is 40%.
- Elevated risk (seven-fold) associated with ectopic pregnancy and the intensity and quantity of PID episodes.
- Dysmenorrhea, profound dyspareunia, irregular menstruation, back pain, malaise, and exhaustion are symptoms of persistent pelvic discomfort.
Diagnosis
It is a clinical diagnosis of PID. Taking into account the possibility of severe morbidity, a low threshold for diagnosis and empirical treatment should be given special consideration for young, sexually active women.
Take into account additional reasons for pelvic pain. - Gonorrhea or chlamydia testing results that are positive confirm the diagnosis; nevertheless, the lack of these diseases does not rule out PID.
- Pherexia >38°C, leucocytosis, and an increased C-reactive protein (CRP) or ESR could all help confirm the diagnosis.
- Although the presence of cervical white cells is non-specific, their absence on microscopy increases the likelihood of PID significantly (negative predictive value 95%).
Investigations - For all women of reproductive age, a pregnancy test including urine or serum β-hCG is necessary (FBC, ESR/CRP).
- HIV testing among other STIs: In case of clinical illness, obtain blood pressure, heart rate, and temperature readings; use NAAT for chlamydia and gonorrhea; if microscopy is available, culture for gonorrhea.
- An ultrasonography scan performed transvaginally can help rule out other conditions including ectopic pregnancy (EP) and show free fluid, thicker dilated tubes, or tuboovarian abscesses.
- Though it is an intrusive procedure and is not always warranted, laparoscopy is regarded as the gold standard diagnostic test.
Management of pelvic inflammatory disease (PID)
General - Consider hospital hospitalization for parenteral therapy and suggest rest if the illness is severe.
- Also recommended is parenteral therapy if there are clinical indications of tubo ovarian abscess or pelvic peritonitis.
- Give the right kind of pain relief.
- Tell them not to have sex until after taking antibiotics, following up, and getting a PN.
- Document the diagnosis, discuss any long-term effects, and emphasize that using barrier contraception in the future will greatly lower the chance of developing PID.
Treatment - Treatment for N. gonorrhoeae, C. trachomatis, and anaerobic infections requires broad range antibiotics.
- Sometimes surgical intervention is necessary. A less intrusive option is ultrasound-guided aspiration of pelvic fluid collections, which can be used for adhesiolysis or to drain pelvic abscesses. One possible procedure during laparoscopy is the division of perihepatic adhesions; however, its potential to enhance the outcome beyond antibiotic therapy is unknown.
- Particular situations :Parenteral therapy is recommended because pregnant women with PID have a higher risk of both fetal and maternal morbidity. Overall, the risks associated with the suggested antibiotic regimens are reasonable; nevertheless, erythromycin should be used instead of tetracyclines.
- Notwithstanding the possibility of more severe PID symptoms, HIV-positive women respond well to routine treatment regimens and don’t need a change in them.
- Ladies sporting an implanted IUD: Expert opinion is divided and there is little evidence to support the removal of an IUD in women with PID.
- With existing PID, continuation of intrauterine contraception is granted a UKMEC 2. It’s not advised to remove the device on a regular basis; removal should only be taken into consideration if the lady asks it or if her symptoms don’t go away after taking antibiotics for 72 hours.
- If the plan is to remove the device, hormonal emergency contraception might be necessary.
Partner notification - Offering gonorrhea and chlamydia screening as well as preventative treatment with a broad-spectrum medication, such as azithromycin 1g stat oral dose, should be extended to current sexual partners.
Though it should be determined by the sexual history, the six-month “look-back” period for PID
Follow-up - To guarantee clinical improvement, review moderate-to-severe disease after 72 hours.
- You should examine mild instances after two to four weeks.
- The purpose of follow-up is to assess PN, compliance with antibiotic therapy, and the remission of symptoms and signs.
- Factors such as non-adherence to medications, enduring symptoms, and the potential for reinfection should be taken into account when determining the necessity of recurrent gonorrhea and chlamydia tests.
You can also read
Reprospot article